The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Study and Its Implications for Patients With Chronic Schizophrenia

- Jonathan Hickman, PMHNP
What do these results mean for a practicing PMHNP?
“Schizophrenia can be a very difficult diagnosis to treat. The CATIE trials provide useful evidence-based data that can guide clinicians in the selection of further pharmacological interventions for patients that are not fully responding to current treatment or experiencing side effects.”
NP Psych Navigator contributors are paid consultants of AbbVie Inc.
Why was the research needed?
Schizophrenia is a challenging psychiatric disorder that is treated using different types of antipsychotic medications. Patients often try older antipsychotic medications (ie, first-generation antipsychotics [FGAs], or typical antipsychotics) and newer medications (ie, second-generation antipsychotics [SGAs], or atypical antipsychotics). However, before the CATIE study, the comparative efficacy and side-effect profiles of these medications were unclear.1,2
While both FGAs and SGAs had been evaluated in clinical trials that demonstrated their efficacy and safety, these trials were short and did not include real-world populations. The trials also did not assess how patients would respond to a wide range of different antipsychotic medications.2
As treatment switches are often made based on patient preference and side effects, research comparing the safety and efficacy of antipsychotic medications in the long term among a diverse population was needed to inform clinical decisions for patients who did not respond to previous treatment.1
What did the researchers do?
The CATIE study, which was funded by the National Institute of Mental Health, was designed to evaluate the effectiveness of conventional (FGAs) and atypical (SGAs) antipsychotics in patients with schizophrenia.1 Adults 18 to 65 years old with schizophrenia who were eligible for oral antipsychotic treatment were enrolled. The study was conducted between January 2001 and December 2004 across 57 clinical sites in the United States.1
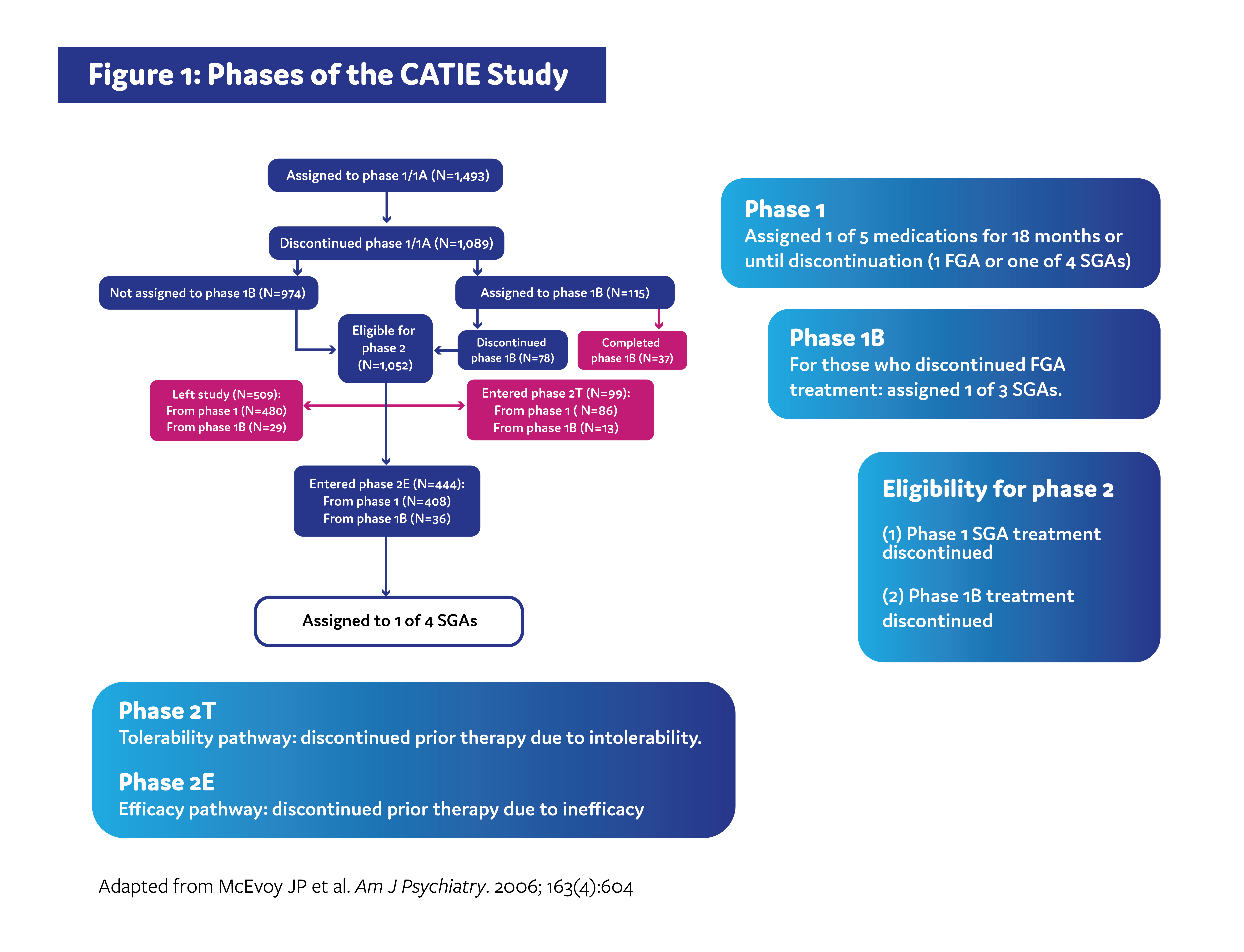
The CATIE study was carried out across multiple phases (Figure 1). In phase 1, patients were randomized to receive either an SGA or an FGA. Patients remained on treatment for 18 months or until discontinuation for any reason. Patients who discontinued treatment in phase 1 due to inadequate symptom control were recommended to enroll in the phase 2 efficacy pathway, and patients who discontinued due to intolerable side effects were recommended to enroll in the phase 2 tolerability pathway. However, patients were ultimately able to choose which pathway to take.1,3
The efficacy pathway was designed to determine if an older SGA would be more effective than SGAs that were newer in comparison, while the tolerability study was designed to determine if any of the 4 SGAs assigned during phase 1 would be beneficial in patients who previously experienced intolerability.3
The primary outcome of both pathways was time until treatment discontinuation for any reason.1,4
What were the key results of the study?
Phase 1 efficacy
Determining efficacy was difficult due to the high rate of treatment discontinuation patients experienced across all medications. That said, both the FGA and SGAs were associated with improved scores on measures of schizophrenia symptom severity and general mental illness severity, although certain medications demonstrated better treatment effects over time than others.4
Phase 1 tolerability
Across all medications, 74% of patients discontinued treatment before the phase 1 study ended at 18 months.5 Across the specific medications, rates of discontinuation ranged from 64% to 82%. However, across medications, there were no significant differences in time to discontinuation due to intolerable side effects. Intolerable side effects that were associated with treatment discontinuation included weight gain, metabolic effects (eg, greater increases in indexes of glucose and lipid metabolism), and extrapyramidal effects.
Phase 2 efficacy
In comparison, patients taking the older SGA stayed on the medication significantly longer than 2 of the 3 groups who received a newer SGA. Fewer patients taking the older SGA discontinued treatment due to lack of efficacy; this difference was significant across all groups. There were no significant differences in discontinuation due to intolerability. Patients taking the older SGA also experienced a greater improvement in symptoms across most measures evaluated.4
Adverse events differed across treatments. Insomnia was most common in those taking an SGA that was relatively newer. Anti-cholinergic symptoms (eg, urinary hesitancy, dry mouth, and constipation) were also most common in another newer SGA. Those taking the older SGA also experienced anti-cholinergic symptoms as well as sialorrhea, and 2 of these patients discontinued treatment due to serious adverse events of eosinophilia and agranulocytosis.4
Phase 2 tolerability
Across the 4 treatment groups, there was a significant difference in time to discontinuation for any reason and a difference in time to discontinuation due to a lack of efficacy. There were no differences in discontinuation across groups due to intolerability. Efficacy analyses revealed a difference in improvement across treatment groups on Positive and Negative Syndrome Scale (PANSS) total scores, the PANSS positive symptom subscale, and the PANSS general psychopathology subscale.1
The number of hospitalizations due to acute exacerbation of schizophrenia, measured per person-years of exposure, was found to differ between the treatment groups. Rates of insomnia, sexual dysfunction, gynecomastia or galactorrhea, orthostatic faintness, weight gain or weight loss, total cholesterol and triglycerides, prolactin levels, and other events occurred at higher rates within some treatment groups when compared with others.1
Why are these results important?
The CATIE study is the largest, longest, and most comprehensive study on how patients with schizophrenia who had previously discontinued therapy responded to different antipsychotic medications over time. Over 1,400 diverse patients were studied across multiple treatment arms at many different clinical sites in the United States, providing important real-world data beyond the scope of the strict criteria required for clinical trials.2
The first phase of the CATIE study found that both an FGA and SGAs were associated with improvements in symptom severity over time. However, because there were some differences in efficacy among the specific medications, individual patients may vary in how they respond to treatment, and clinicians should take an individualized approach to prescribing these medications. The second phase of CATIE found that an older SGA might be beneficial for patients who discontinued previous antipsychotic medications due to inefficacy. However, in both phases, treatment discontinuation was high, limiting researchers’ ability to draw firm conclusions about the efficacy of the medications studied.1,5
In phase 2, it was discovered that switching to a different SGA may not provide an efficacy advantage in those who discontinued due to intolerability. These findings suggest that careful consideration of a patient’s medication history may provide helpful guidance on the advantages of a possible switch.1,3,4
As a whole, the patients in these studies discontinued treatment at high rates, indicating limitations to the treatment options studied. These findings highlight a significant unmet need in optimal management of chronic schizophrenia.1
Lastly, it is important to take the limitations of this study into account. Sample sizes became quite small as the trials went on, which may have significantly impacted statistical findings. In addition, since this study was published, other medications with different mechanisms of action have become available.1,4
What’s next?
The CATIE study provided important evidence to help guide treatment switches in patients with schizophrenia who have discontinued previous therapies due to inefficacy or intolerability.
Additional data are needed to better understand the comparative efficacy and safety of newer medications.1
Greater exploration of cost-effectiveness, quality of life, and predictors of response across different antipsychotic medications could provide important information for clinicians on when to make a treatment switch for patients with schizophrenia.2
References
Stroup TS, Lieberman JA, McEvoy JP, et al. Am J Psychiatry. 2006;163(4):611- 622.
- National Institute of Mental Health. Questions and answers about the NIMH Clinical Antipsychotic Trials of Intervention Effectiveness Study (CATIE) — phase 1 results. 2005.
- National Institute of Mental Health. Questions and Answers About the NIMH Clinical Antipsychotic Trials of Intervention Effectiveness Study (CATIE) — Phase 2 Results. 2006.
- McEvoy JP, Lieberman JA, Stroup TS, et al. Am J Psychiatry. 2006;163(4):600- 610.
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-23.
This summary was prepared independently of the study’s authors.
This resource is intended for educational purposes only and is intended for US healthcare professionals. Healthcare professionals should use independent medical judgment. All decisions regarding patient care must be handled by a healthcare professional and be made based on the unique needs of each patient.
ABBV-US-00739-MC, Version 2.0
Approved 01/2024
AbbVie Medical Affairs
