Skip to section
Introduction to Psychopharmacology
Psychopharmacology explores how neurotransmitters in the nervous system affect physiological processes. Neurotransmitters can have diverse effects on physiological processes including mood, behavior, cognition, and movement. Abnormal levels of neurotransmitters are thought to play a role in various diseases, including major depressive disorder (MDD), bipolar I disorder, and schizophrenia. The study of psychopharmacology aims to understand neurotransmitter imbalances and the potential effects therapeutic agents may have.1,2
Visualizing the synthesis, localization, and metabolism of certain neurotransmitters may help to understand how various symptoms can manifest in psychiatric disorders. Healthcare providers can learn how these neurotransmitters are thought to be modulated to better understand the potential impact they may have in psychiatry.1
*Please note that this article provides only a theoretical overview, and the clinical relevance of pre-clinical studies is unknown.
Neurotransmitters
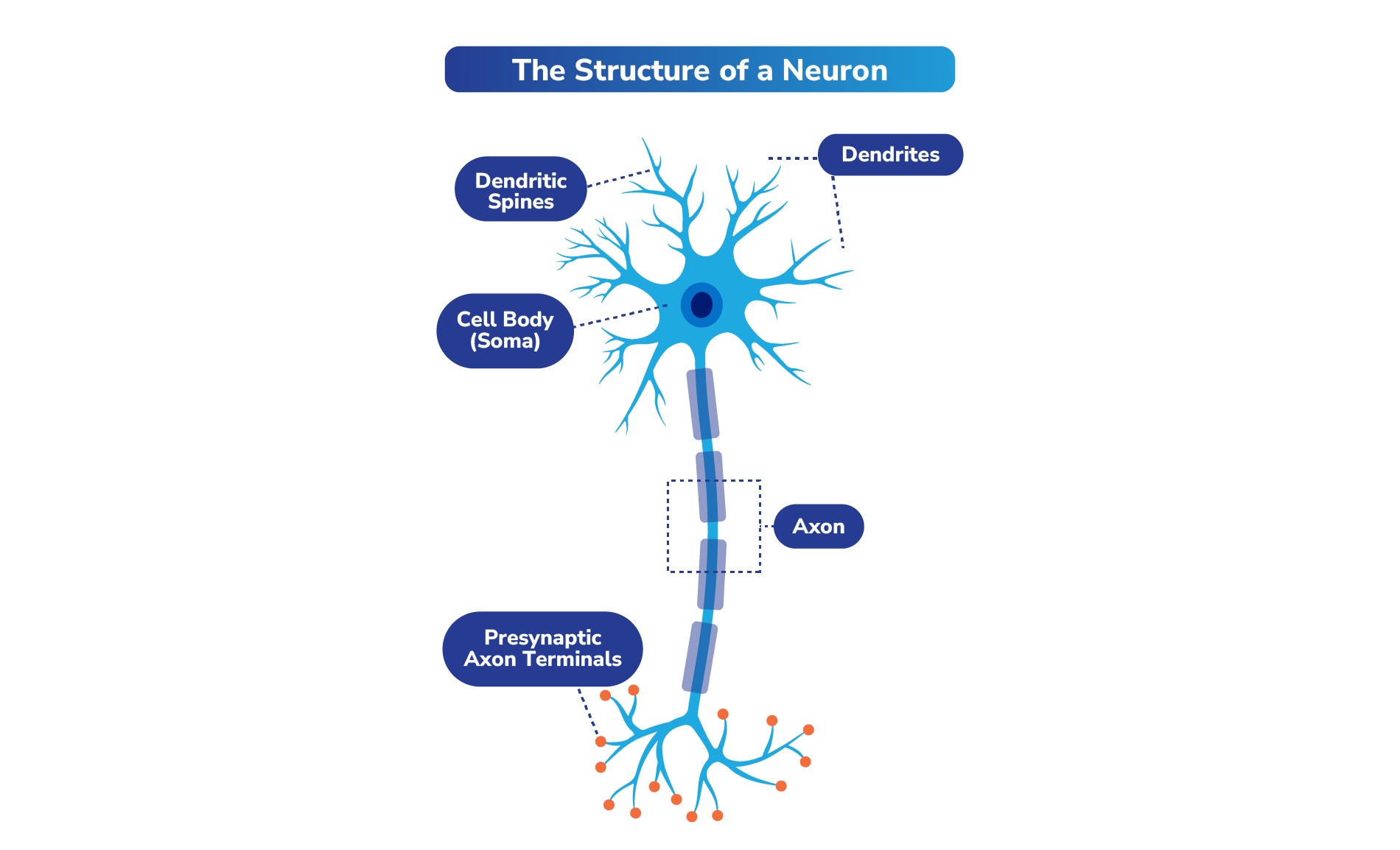
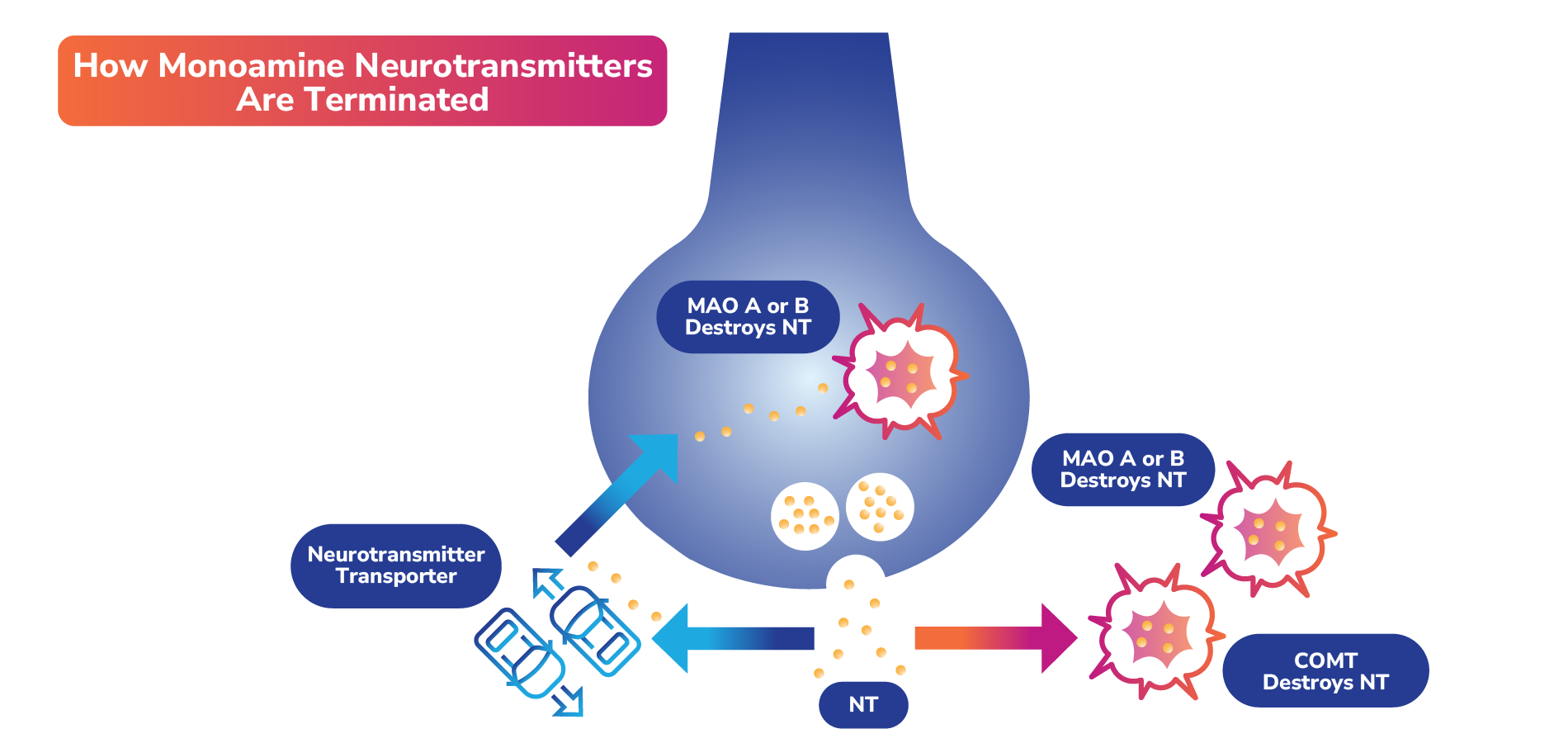
Neurotransmitters are chemical signaling molecules in the nervous system that are transmitted through neurons (cells in the nervous system, or nerve cells). It has been estimated that an average of 86 billion neurons make up the brain, forming a network of connections responsible for our thoughts, emotions, and actions.3
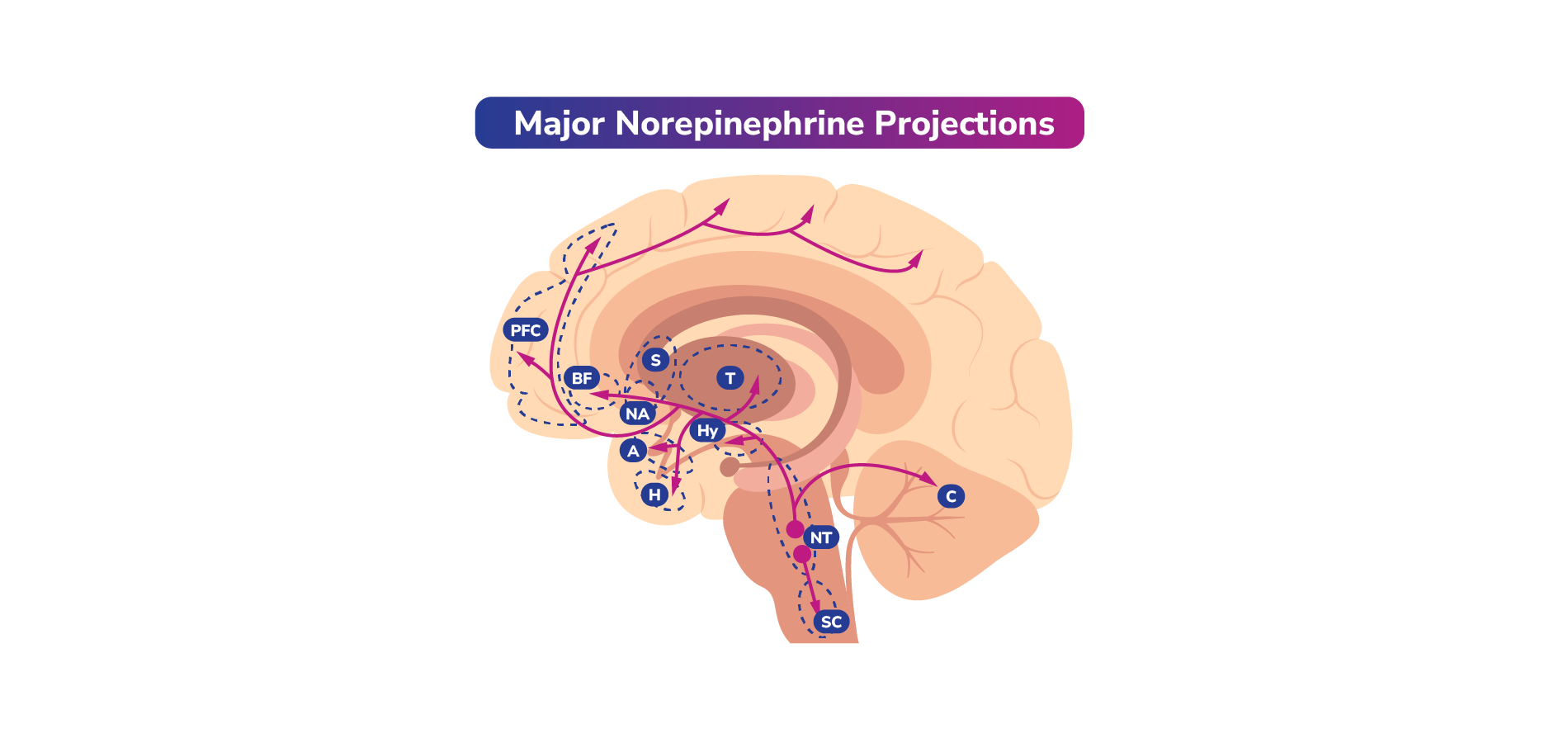
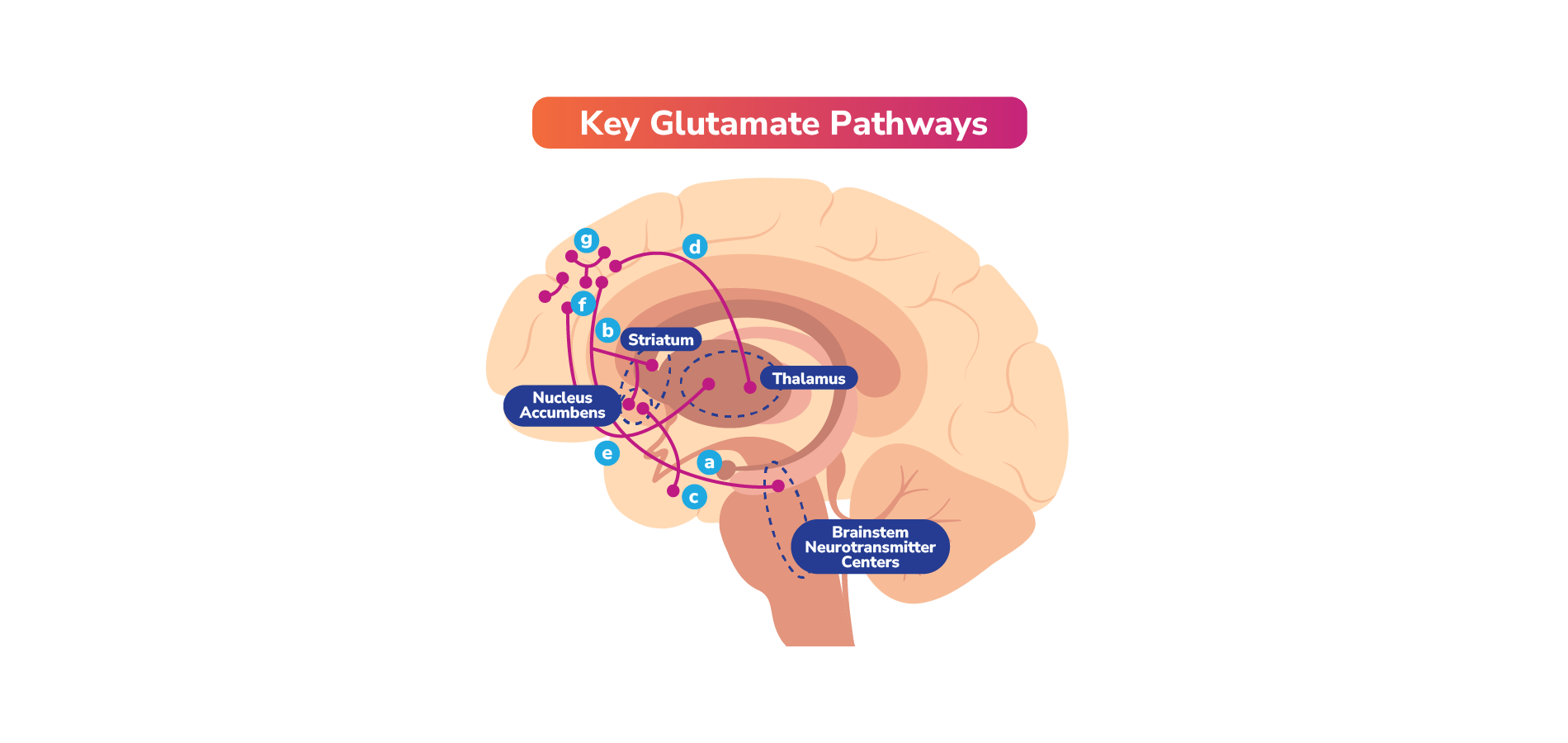
Select Neurotransmitter Pathways and Receptors That May Play a Role in Schizophrenia, Bipolar I Disorder, and/or MDD
Dopamine
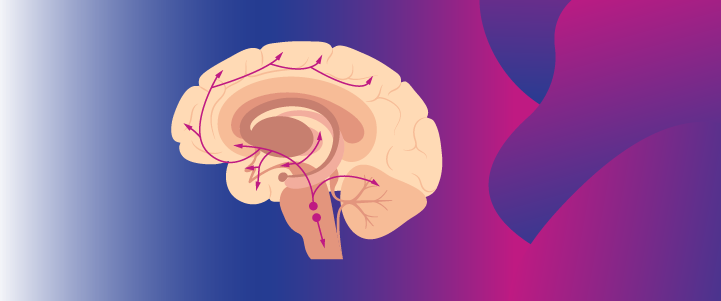
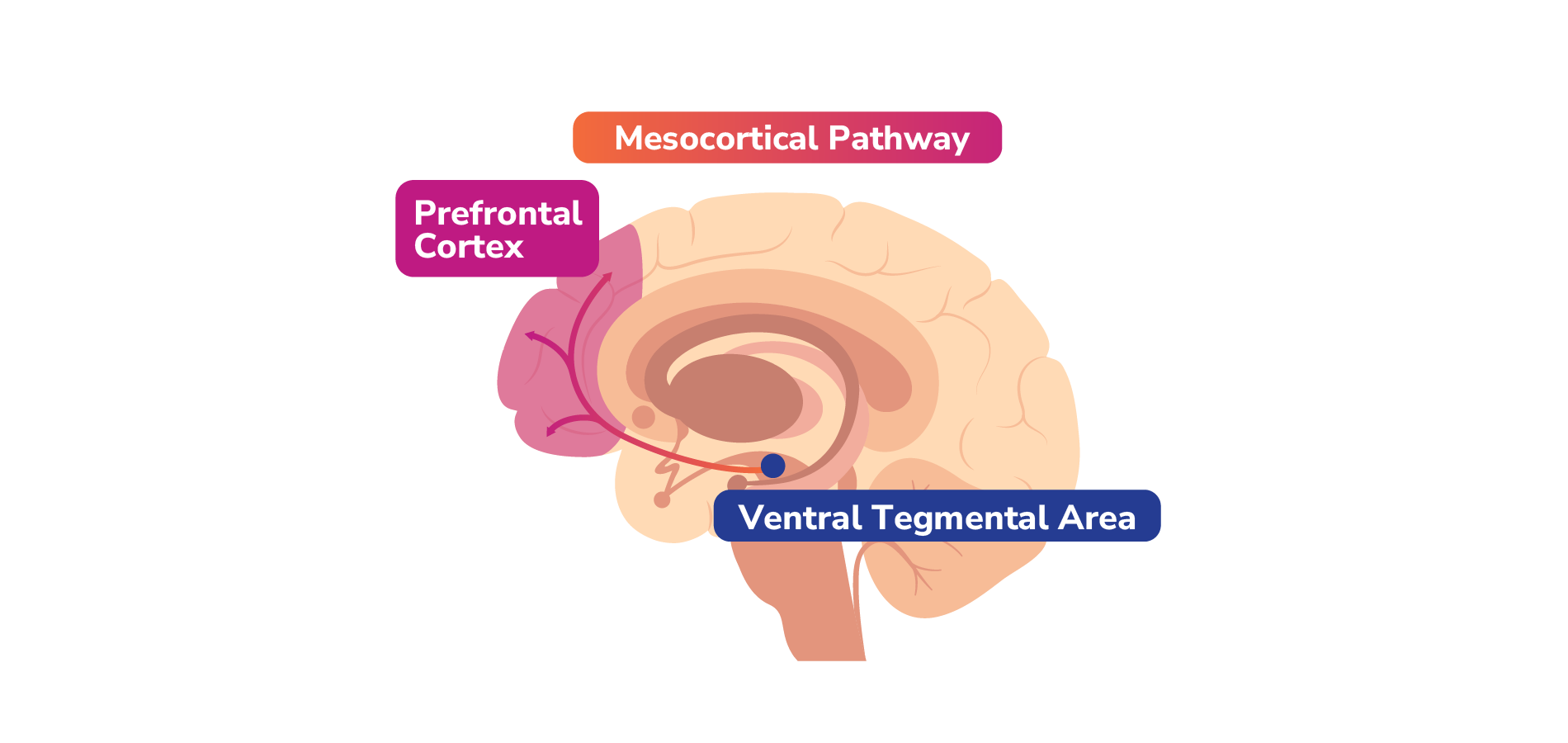
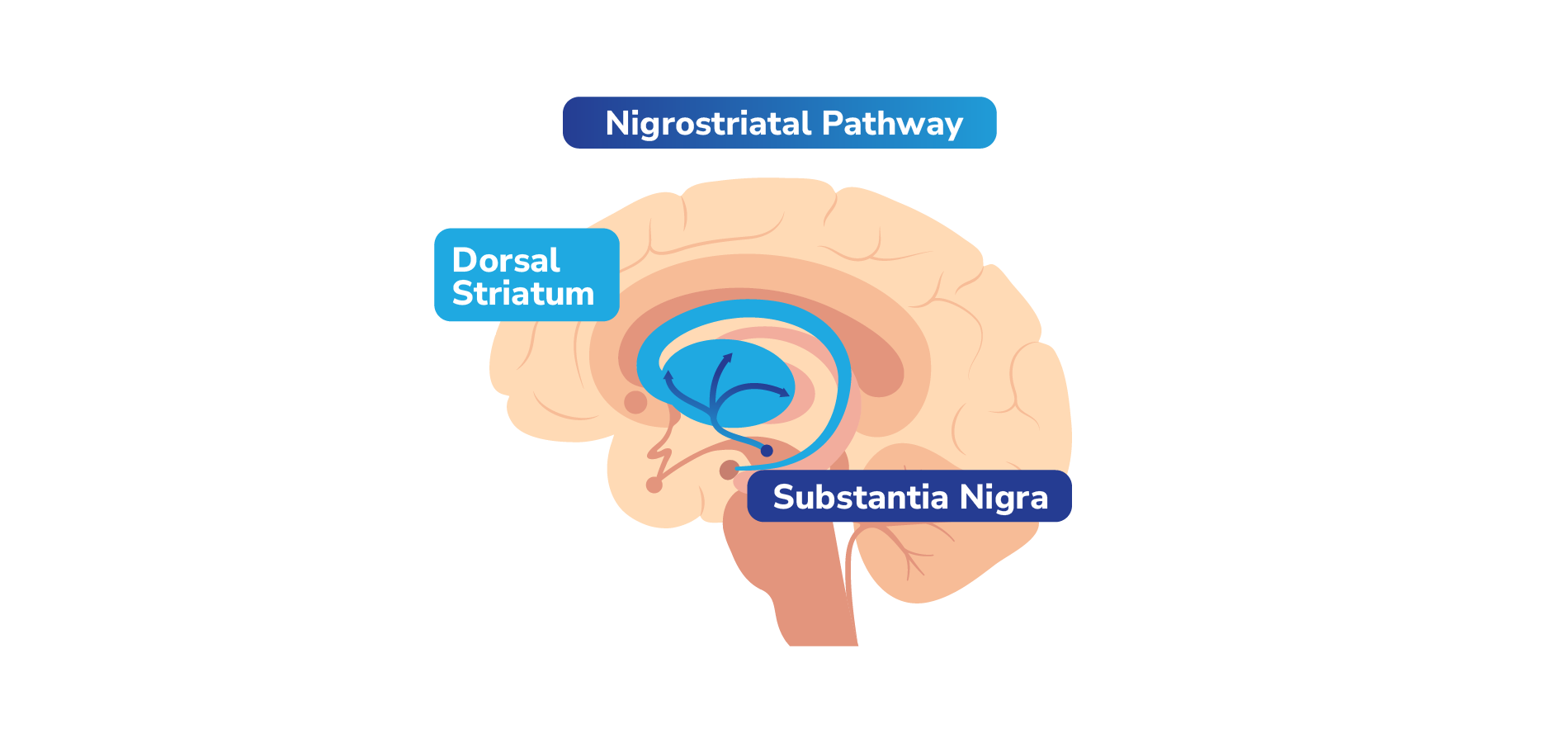
Dopamine is a neurotransmitter produced in the dopaminergic neurons located in the substantia nigra and ventral tegmental area (VTA).10 In preclinical studies, it has been shown to be involved in the regulation of a range of physiological functions of the CNS, including motor control, feeding, affect, reward, sleep, memory, learning, and cognition.2,11
Dopamine Pathways
Dopamine travels to different parts of the brain through 4 key pathways, each with different functions.1
Mesolimbic pathway: Projects dopaminergic neurons from the VTA to the nucleus accumbens (NAc) in the ventral striatum.1
Interplay Between Neurotransmitters (Examples)
Norepinephrine, dopamine, and serotonin often work together. Dysfunction of these neurotransmitters is hypothesized to play a role in the symptoms associated with mood disorders. Thus, certain medications utilized for psychiatric conditions such as MDD or bipolar disorder can often impact one or more of these neurotransmitter pathways.1
The interactions between dopamine and serotonin are of particular importance in helping to regulate mood, cognition, and motor function and will be discussed here. Specifically, activation or inhibition of serotonin receptors can modulate the release of dopamine downstream.
Mechanisms of Actions and Impact of Medications That May Affect Neurotransmitter Signaling
Medications may alter behavior by reducing or enhancing activity at the receptor.
Agonists (↑): Agonists are compounds that bind to receptors and activate them to the full extent. They can mimic the action of the neurotransmitter and can be used to increase neurotransmitter activity when it is deficient.1
Antagonists (X): Antagonists bind to receptors but do not activate them. Instead, they block the receptor and prevent it from being activated by agonists or neurotransmitters, thus blocking the agonist-mediated response. Antagonists can be used to inhibit excessive neurotransmitter activity or block unwanted effects of agonists.1
Partial Agonists (~): Partial agonists can act as functional agonists or functional antagonists depending on the surrounding levels of naturally occurring neurotransmitters. For example, in the absence of a full agonist, a partial agonist can demonstrate agonist activity and activate the receptor. However, the response elicited by a partial agonist will be lower than that of a full agonist. In the presence of a full agonist, however, a partial agonist can act as an antagonist, preventing the receptors from being activated.40
Partial agonists have a lower intrinsic activity than full agonists. When they bind to receptors, they produce a response that is less than that of a full agonist. The activity of partial agonists at a receptor lies between that of agonists and antagonists.40 Partial agonists can be useful in situations where a full response is not desired.1
Example Actions and Potential Downstream Effects of Drugs Modulating Dopamine and Serotonin Activity
Dopamine and Serotonin Receptor Antagonism
EPS Modulation
Antagonism of both D2 and 5-HT2A receptors can release the inhibition on dopamine release and lead to an increase in dopamine concentration in the striatum. Increased dopamine release in the striatum due to 5-HT2A antagonism can allow dopamine to compete with the D2 receptor antagonists. This may help reduce the risk of EPS. Conversely, activation of 5-HT1A receptors and the resulting release of dopamine in the striatum is believed to play a role in mitigating the potential EPS that may occur due to D2 antagonism.1
Prolactin Regulation
Serotonin and dopamine have opposite roles in regulating prolactin secretion. Dopamine can inhibit prolactin release by stimulating the D2 receptors. Serotonin promotes prolactin release via activation of the 5-HT2A receptors. If the D2 receptors are antagonized, dopamine binding may be disrupted, and prolactin levels may increase. However, if 5-HT2A receptors are blocked in addition to D2 receptors, this may increase dopamine levels, inhibit prolactin release, and potentially help reduce the hyperprolactinemia caused by the D2 receptor blockade alone.1
General Clinical Safety and Monitoring Considerations for Psychopharmacologic Agents
It is important to note that there are patient-dependent factors that may affect response to psychopharmacological treatment.